| Disease | Alb | PZQ | IVM | AZM | DEC | MOX | Other |
|---|---|---|---|---|---|---|---|
| STH | ✓ | MBD | |||||
| Schisto | ✓† | ✓ | OXA | ||||
| LF | ✓† | ✓ | ✓ | ✓ | DOX* | ||
| Trachoma | ✓‡ | ✓ | TEO |
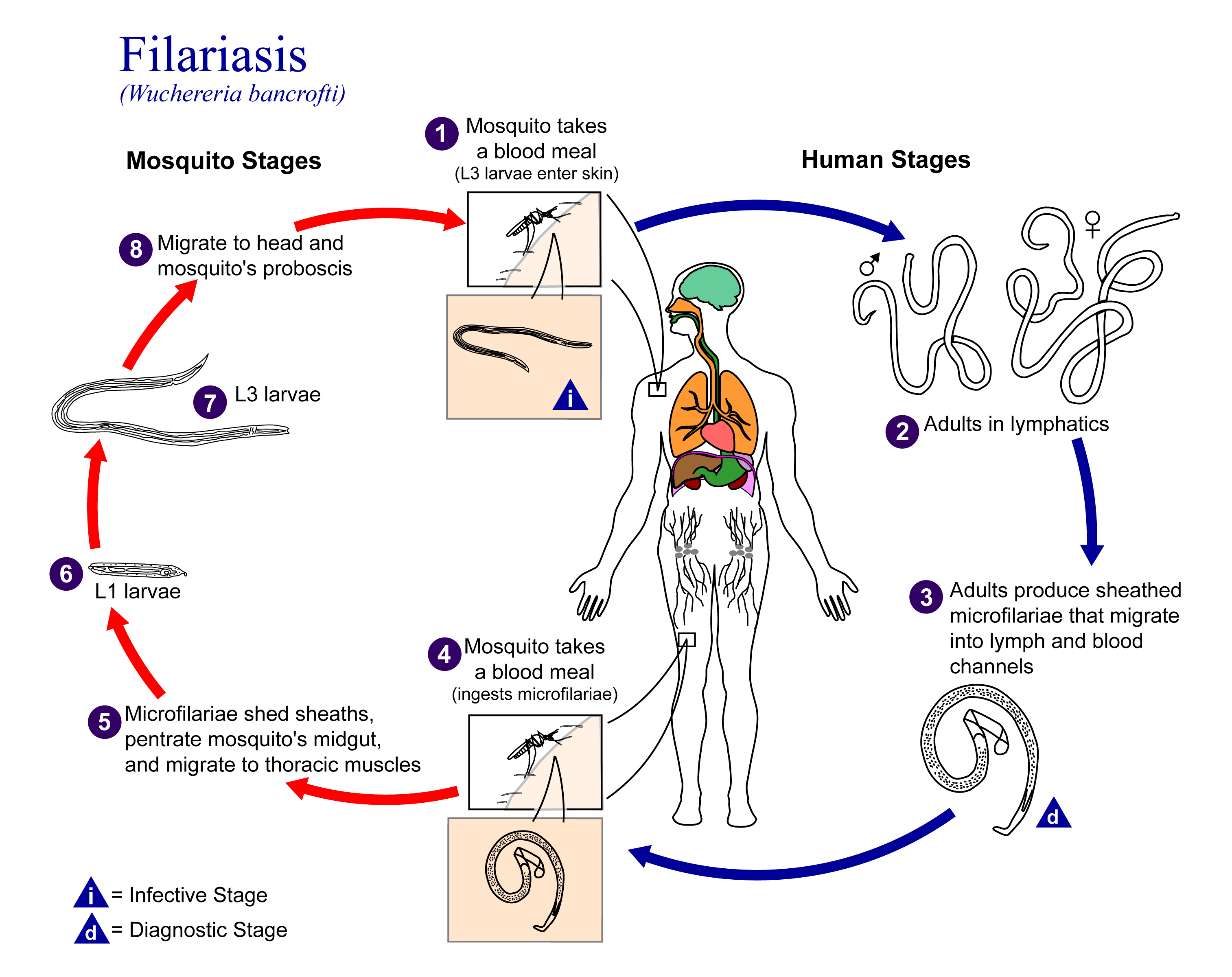
Accounting for the impact of mass-drug administration for NTDs prevalence mapping using geostatistical methods
A decay-adjusted spatio-temporal (DAST) model
Lancaster University
Presentation Overview
- Neglected Tropical Diseases and Mass Drug Administration
- The standard geostatistical model for prevalence mapping
- A decay-adjusted spatio-temporal model
- Application to lymphatic filariasis in Madagascar
- Summary
Neglected tropical diseases
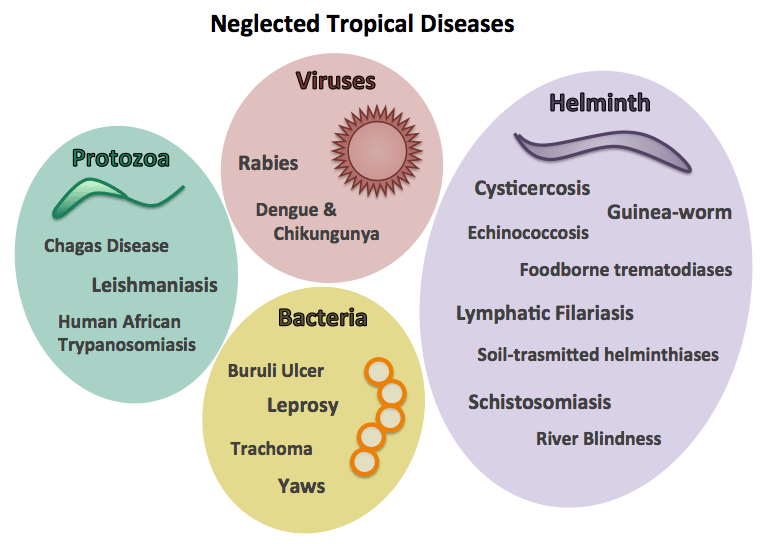
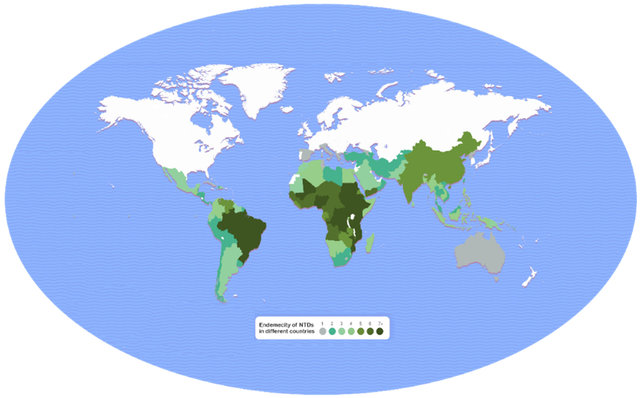
NTD endemic areas
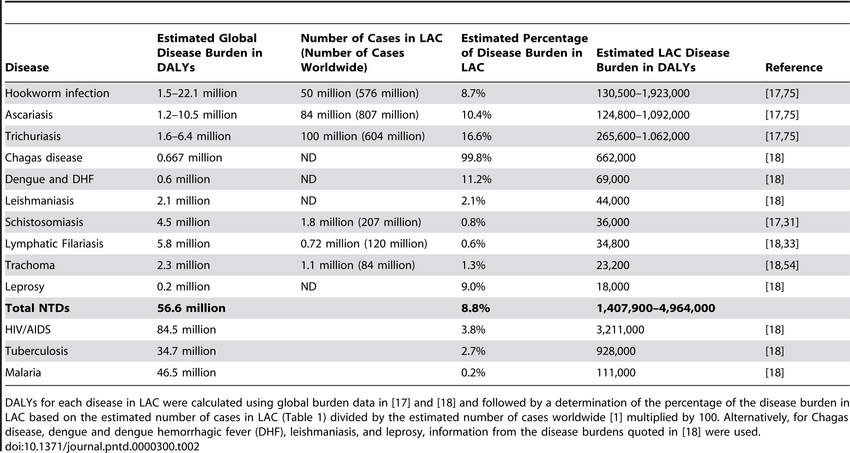
The impact of NTDs
NTDs Risk Factors


Common risk factors:
- Poor sanitation and lack of clean water
- Limited healthcare access
- Poverty and overcrowding
- Exposure to disease vectors (e.g., mosquitoes, flies)
- Lack of education and awareness
Mass Drug Administration (MDA): A Preventive Strategy
What is MDA? Mass Drug Administration involves the periodic distribution of safe, effective medications to entire at-risk populations—regardless of disease status—to reduce transmission and prevent infections.
Why is it used?
Controls or eliminates disease in endemic areas
Reduces the community-level parasite burden
Prevents long-term complications and disability
Key Features:
Delivered at regular intervals (annually or biannually)
Often combined with public health education
Supported by WHO and major global health initiatives
Albendazole

How it works:
Albendazole disrupts the metabolism of parasitic worms by inhibiting microtubule formation, which is essential for their survival.
Used for NTDs such as:
Lymphatic filariasis (in combination with ivermectin or DEC)
Soil-transmitted helminths (e.g., ascariasis, hookworm, trichuriasis)
MDA use:
Distributed in large-scale deworming campaigns, particularly targeting children and at-risk communities.
Ivermectin
How it works:
Ivermectin paralyzes and kills parasites by interfering with their nerve and muscle function via chloride channel disruption. Parasites cannot move and feed.
Used for NTDs such as:
Onchocerciasis (river blindness)
Lymphatic filariasis (in combination with albendazole)
Strongyloidiasis
MDA use: Ivermectin is given to both adults and children (weighing more than 15kg), but its use is carefully considered for pregnant women.
Praziquantel
How it works:
Praziquantel increases permeability of the parasite’s cell membranes to calcium ions, causing muscle contraction and paralysis.
Used for NTDs such as:
Schistosomiasis
Tapeworm infections
MDA use:
Key drug in school-based and community-wide treatment campaigns in regions with high schistosomiasis burden.
Treating NTDs
Abbreviations:
Alb = Albendazole, PZQ = Praziquantel, IVM = Ivermectin, AZM = Azithromycin, DEC = Diethylcarbamazine, MOX = Moxidectin, MBD = Mebendazole, OXA = Oxamniquine, DOX = Doxycycline, TEO = Tetracycline eye ointment
Symbols:
† Used in combination therapy
‡ Only in onchocerciasis co-endemic areas
*Adjunctive treatment
Assessing MDA Impact
Timeframe for observable impact:
STH: Reduced prevalence seen after 1-2 annual rounds
Schistosomiasis: Egg reduction visible after 1-2 years
LF: Microfilariae reduction within months, but breaking transmission requires 5+ years
Trachoma: Active disease reduction in 1-3 years
Monitoring surveys:
Baseline surveys (pre-MDA)
Transmission Assessment Surveys (TAS) for LF after 5+ rounds
Impact surveys (after 3-5 rounds)
Post-treatment surveillance (after reaching elimination targets)
Key indicators:
Parasitological prevalence (STH, schisto)
Antigenemia (LF)
TF/TI rates (trachoma)
Entomological indices (for vector-borne NTDs)
Modelling the impact of MDA
The standard geostatistical model for prevalence mapping
- Outcome: \(Y_i\) number of cases out \(n_i\) sampled
- Locations: \(X = (x_1, \ldots, x_n)\)
- Covariates (optional): \(d(x)\) (e.g. elevation, distance from waterways)
- Spatial Gaussian process: \(S(x)\) (stationary and isotropic) \[ {\rm cov}\{S(x), S(x')\} = \sigma^2 \rho(||x - x'||; \phi) \] Example: \(\rho(u; \phi) = \exp\{-u/\phi\}\).
- Conditional independence: \(Y_{i}\) conditionally on \(S(x_i)\) are mutually independent \(Bin(n_i , p(x_i))\)
- The linear predictor \[ \log\left\{\frac{p(x_i)}{1-p(x_i)}\right\} = \beta_0 + d(x_i)^\top \beta + S(x_i) \]
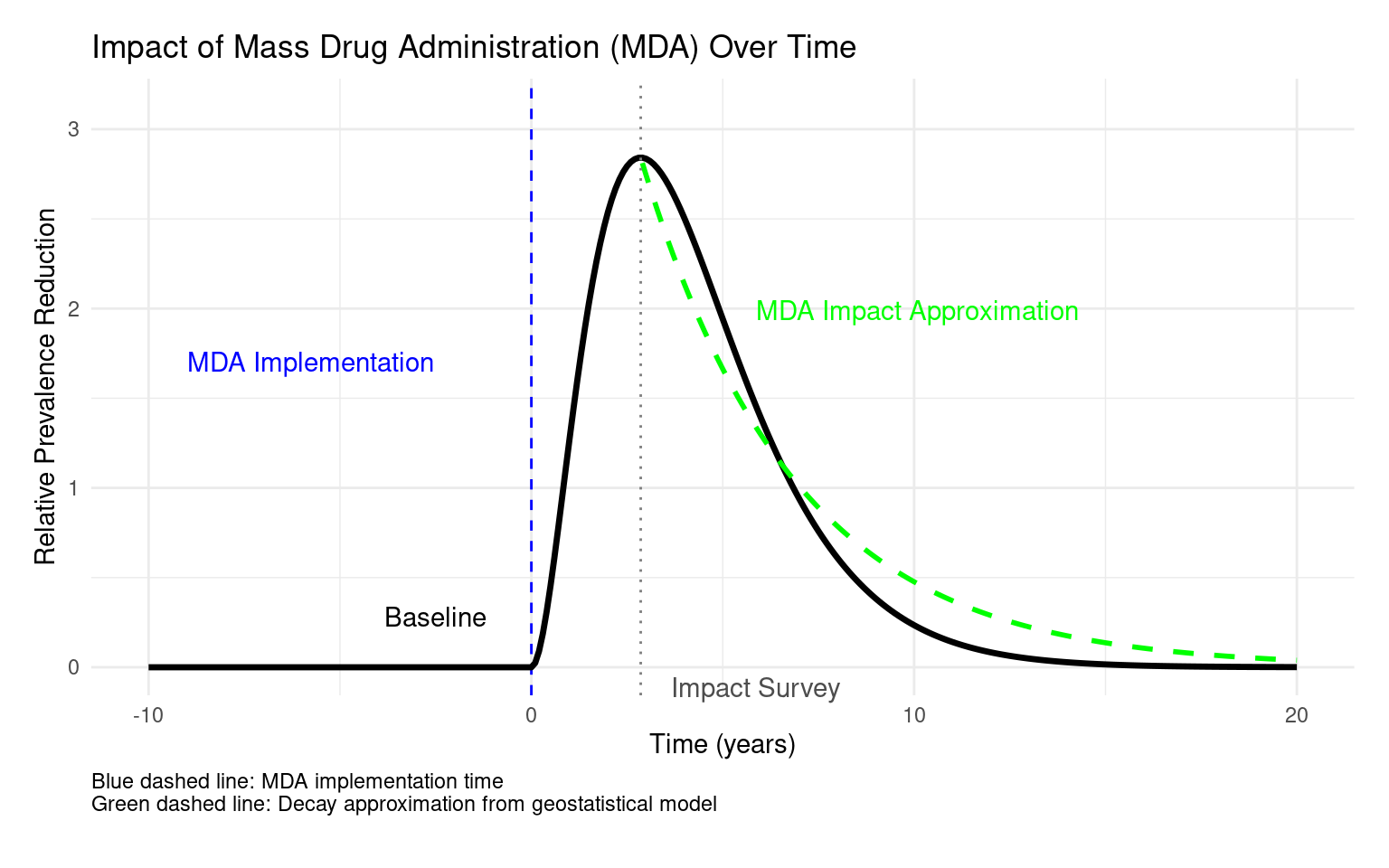
Spatial and temporal confounding with MDA
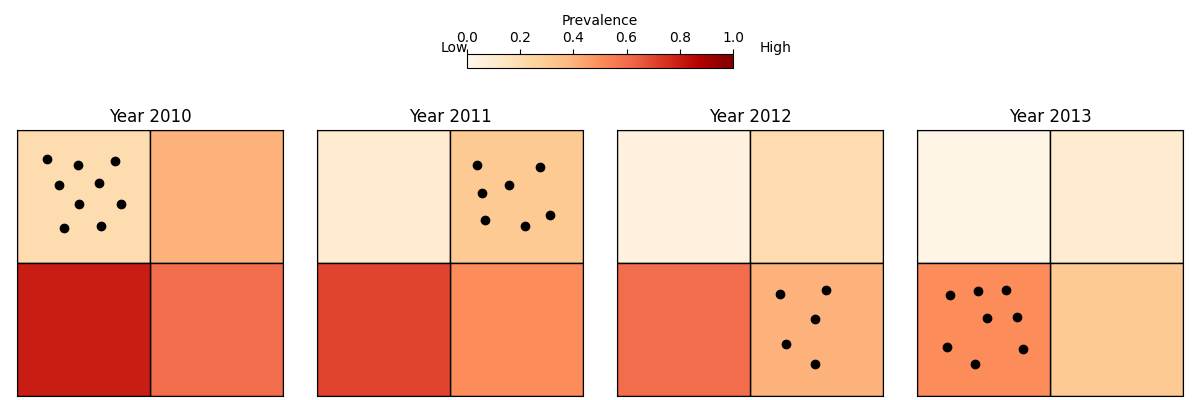
Issues:
Areas with different levels of transmission are sampled at different times.
Use of MDA rounds can even show a positive associated with disease risk
Modelling the Impact of MDA
We model observed prevalence \(P(x,t)\) as a product of:
- Counterfactual prevalence, \(P^*(x,t)\), in the absence of MDA
- A cumulative MDA effect, based on rounds of effective treatment
\[ P(x, t) = P^*(x, t)\prod_{j=1}^{r(t)} \left[1 - f(t - u_j)\right]^{I(x, u_j)} \]
- \(u_j\): times of effective MDA rounds
- \(I(x, u_j)\): indicator of MDA coverage at location \(x\) and time \(u_j\)
- \(r(t)\) is the number of MDA rounds carried out before time \(t\)
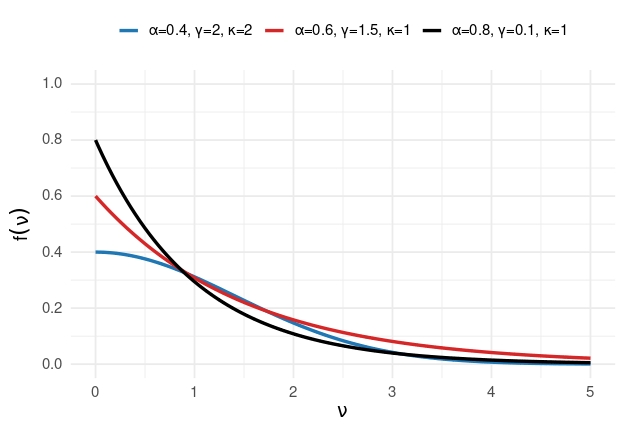
Parametric form of MDA decay function
\[ f(v) = \alpha \exp\left\{ -\left(\frac{v}{\gamma}\right)^{\kappa} \right\} \]
- \(\alpha\): maximum reduction immediately after MDA
- \(\gamma\): scale of decay
- \(\kappa\): shape of decay (fixed)
Monte Carlo Maximum Likelihood
- Let \(W_i = S(x_i) + Z_i\) and \(W = (W_1, \ldots, W_n)\)
The likelihood function for parameters \(\theta = (\beta, \sigma^2, \phi, \tau^2)\) is given by:
\[ L(\theta) = \int N(W; D\beta, \Omega) f(y| W) dW \]
where \(\Omega = \sigma^2 R(\phi) + \tau^2I_n\).
We approximate this using Monte Carlo integration:
\[ L_m(\theta) = \frac{1}{B} \sum_{j=1}^{B} \frac{N\left(W^{(j)}; D\beta, \Omega \right)}{N\left(W^{(j)}; D\beta_0, \Omega_0\right)} \] where \(W^{(i)}\) are sampled from the distribution of \(W\) given \(y\) using an MCMC algorithm.
All implemented in the
dastfunction of theRiskMappackage
Applications
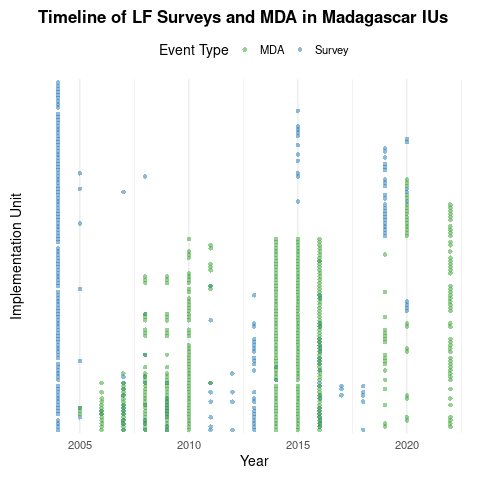
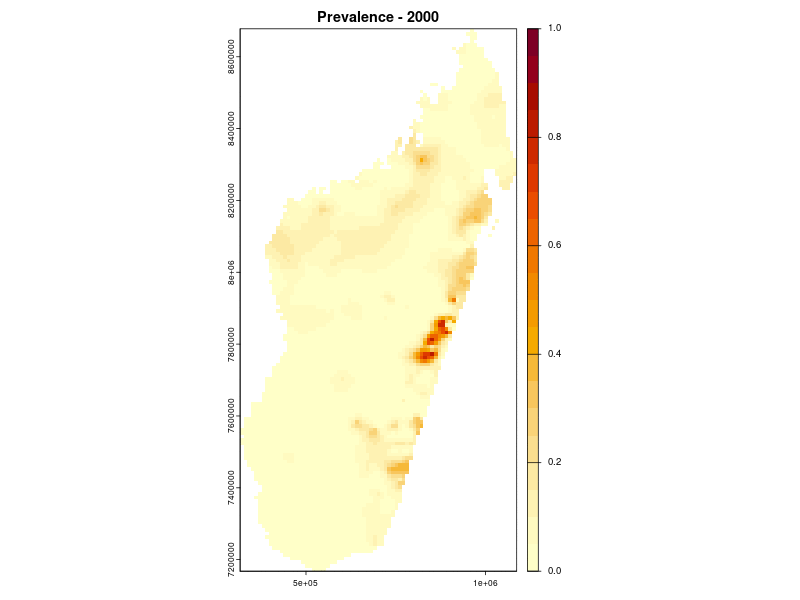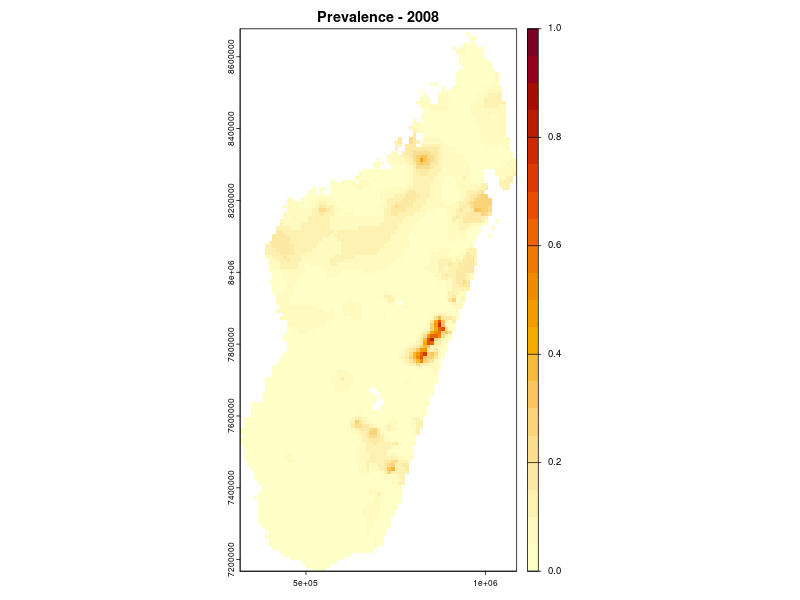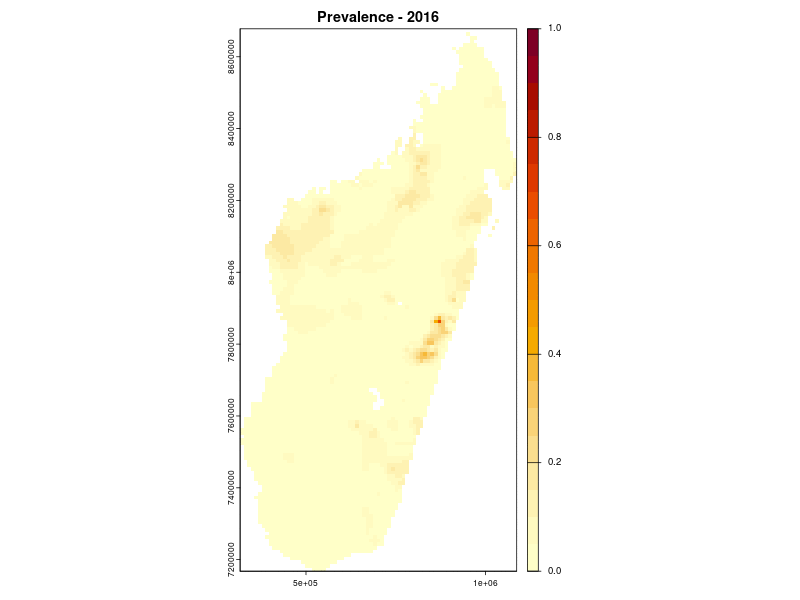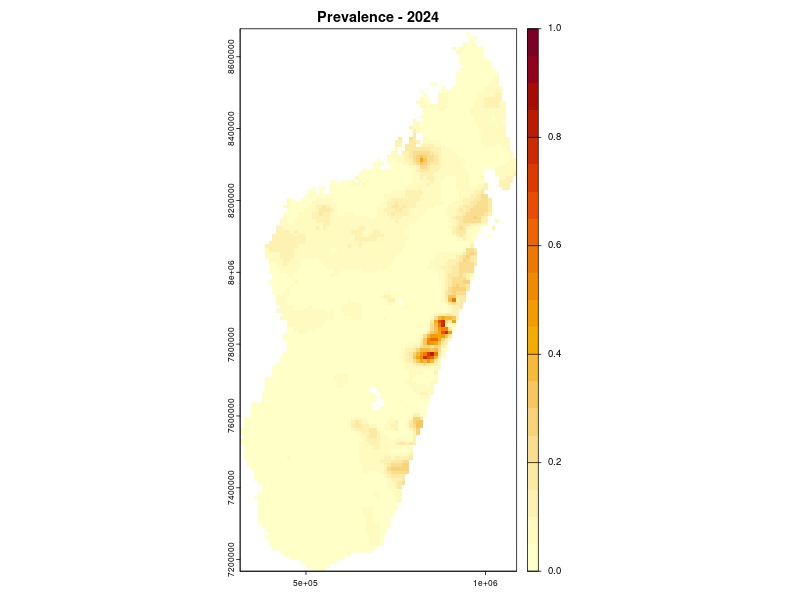
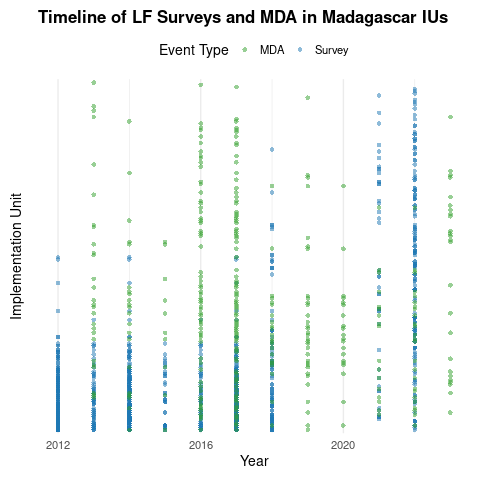
Example: Lymphatic filariasis in Madagascar
The data
Timeline of surveys and MDA
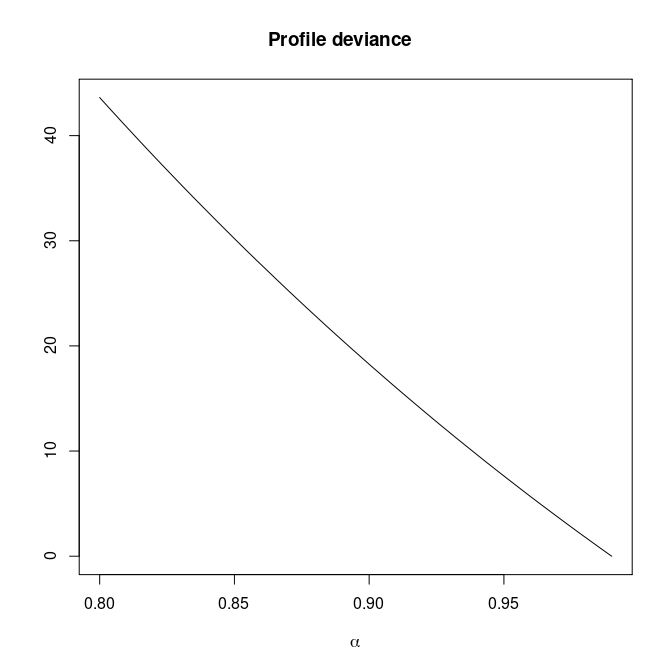
Monotone likelihood for \(\alpha\)
Introducing penalization for \(\alpha\)
- Let \(p(\alpha) > 0\) be a positive function.
- The penalized likelihood for \(\alpha\) is \[ L_{p}(\theta) = L(\theta) - p(\alpha) \]
Proposed penalty
\[ p(\alpha) = -\left[\lambda_1 \log\alpha + \lambda_2\log(1-\alpha) \right] \]
- We set \(\lambda_1 = 20\) and \(\lambda_2 = 14\). (This inspired by a Beta distribution with mode in around 0.6, with 0.42 and 0.7 as its 0.025 and 0.975 quantiles, respectively.)
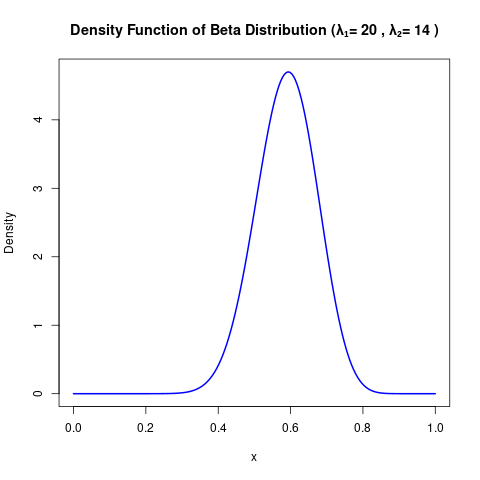
Introducing penalization for \(\alpha\)
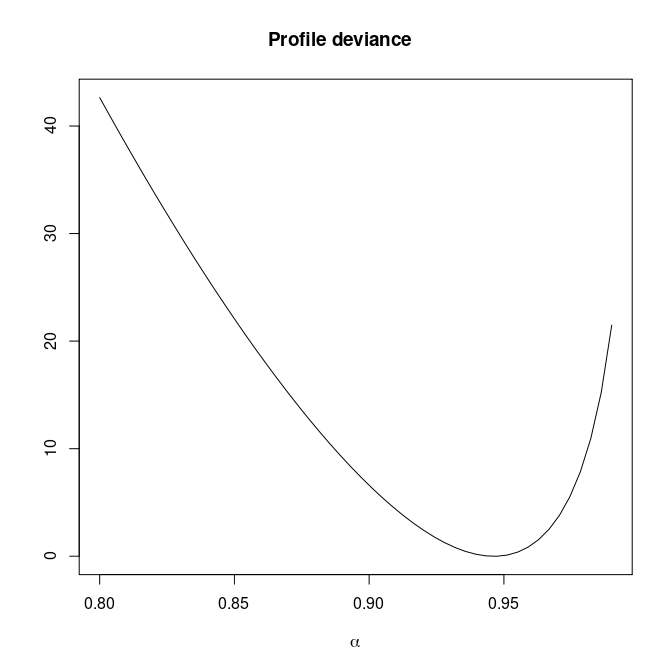
Paramter estimates
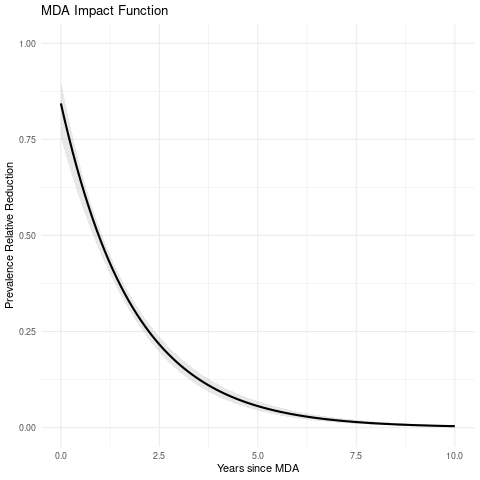
MDA impact function: \(f(v) = \alpha\exp\{-v/\gamma\}\), with \(v\) denoting the years since last MDA
| Parameter | Estimates |
|---|---|
| Intercept | -2.582 (-2.622, -2.541) |
| Spatial variance | 2.204 (1.891, 2.569) |
| Spatial scale | 79.153 (70.874, 88.399) |
| Max. reduction (\(\alpha\)) | 0.856 (0.839, 0.872) |
| Decay scale (\(\gamma\)) | 1.802 (1.768, 1.837) |
Estimated MDA impact function
Prevalence prediction 2000-2024
Example: soil-transmitted helminths in Kenya
The data
Timeline of surveys and MDA
Paramter estimates
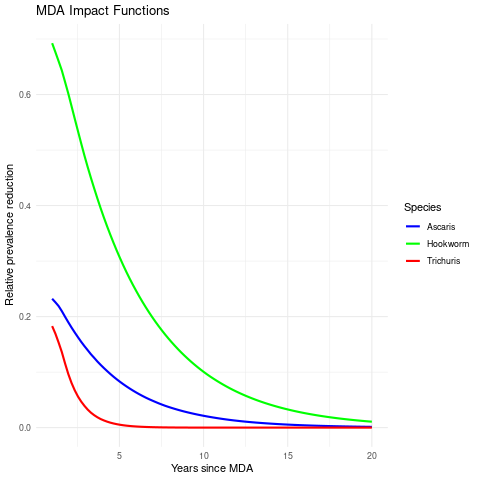
MDA impact function: \(f(v) = \alpha\exp\{-v/\gamma\}\), with \(v\) denoting the years since last MDA
| Parameter | Ascaris | Trichuris | Hookworm |
|---|---|---|---|
| Intercept | -2.41 (-2.45, -2.37) | -2.44 (-2.51, -2.37) | -1.94 (-1.98, -1.90) |
| Spatial variance | 26.83 (14.70, 48.97) | 15.57 (8.35, 29.06) | 29.75 (20.17, 43.89) |
| Spatial scale | 263.31 (178.38, 388.66) | 109.02 (72.34, 164.32) | 198.63 (152.33, 258.99) |
| Max. reduction (\(\alpha\)) | 0.318 (0.299, 0.338) | 0.837 (0.533, 0.958) | 0.892 (0.871, 0.909) |
| Decay scale (\(\gamma\)) | 9.49 (7.41, 12.16) | 1.23 (1.01, 1.49) | 6.46 (6.06, 6.90) |
The MDA impact by STH species
Prevalence prediction 2012-2023
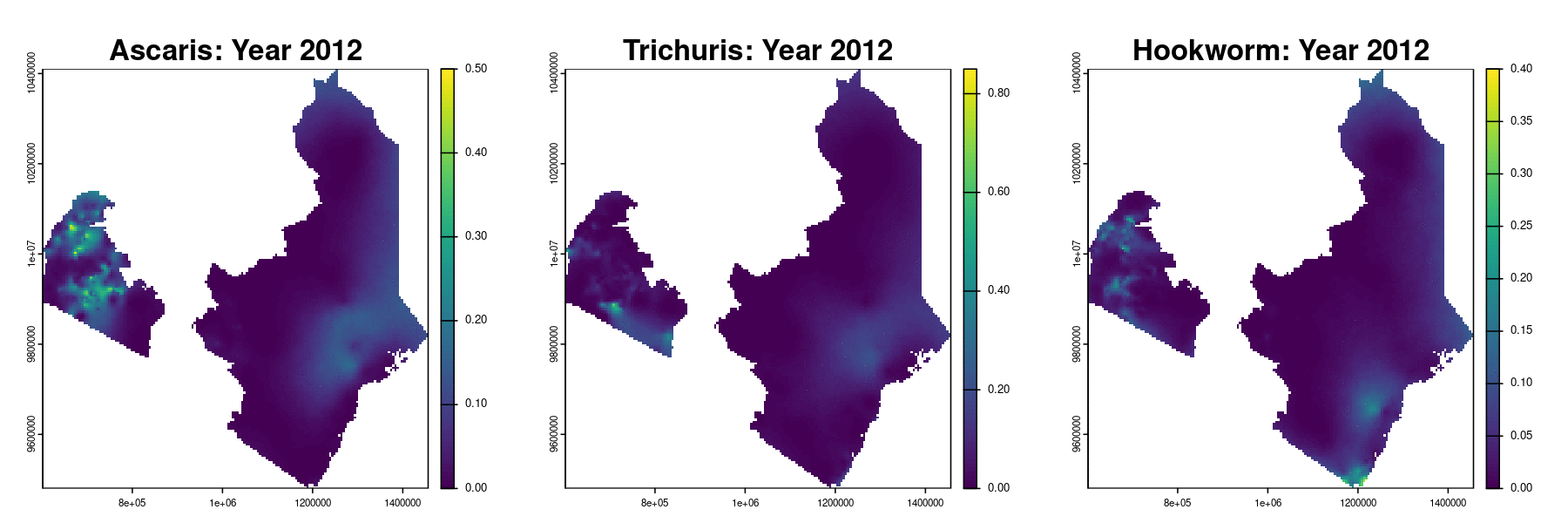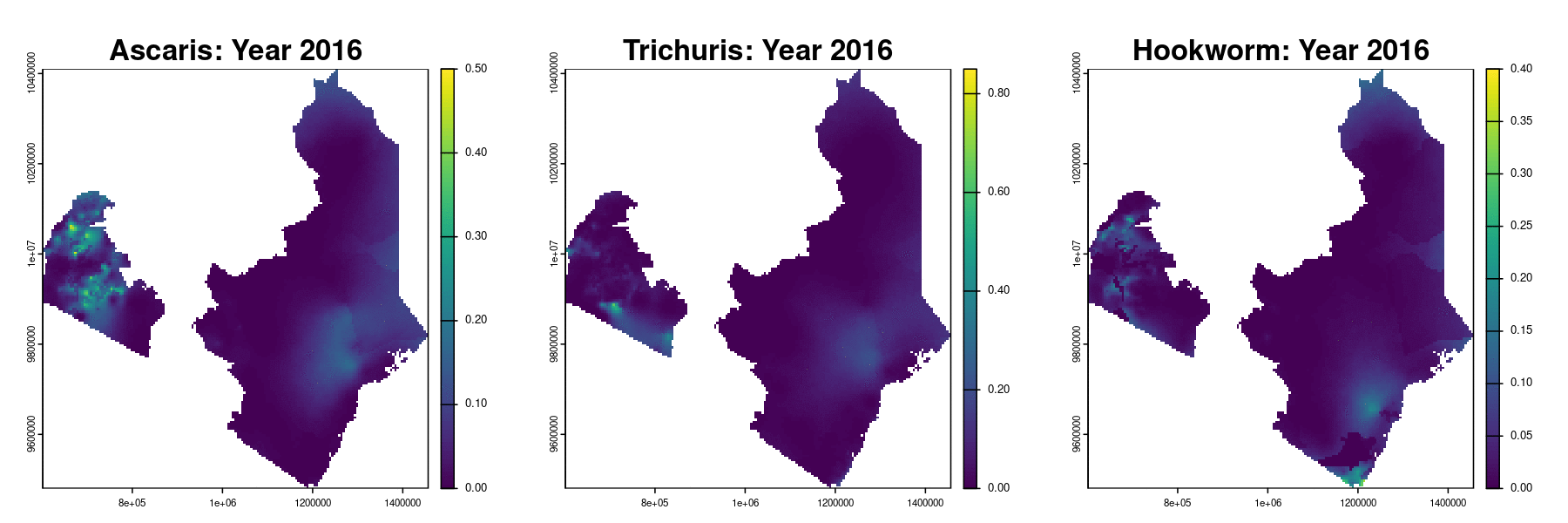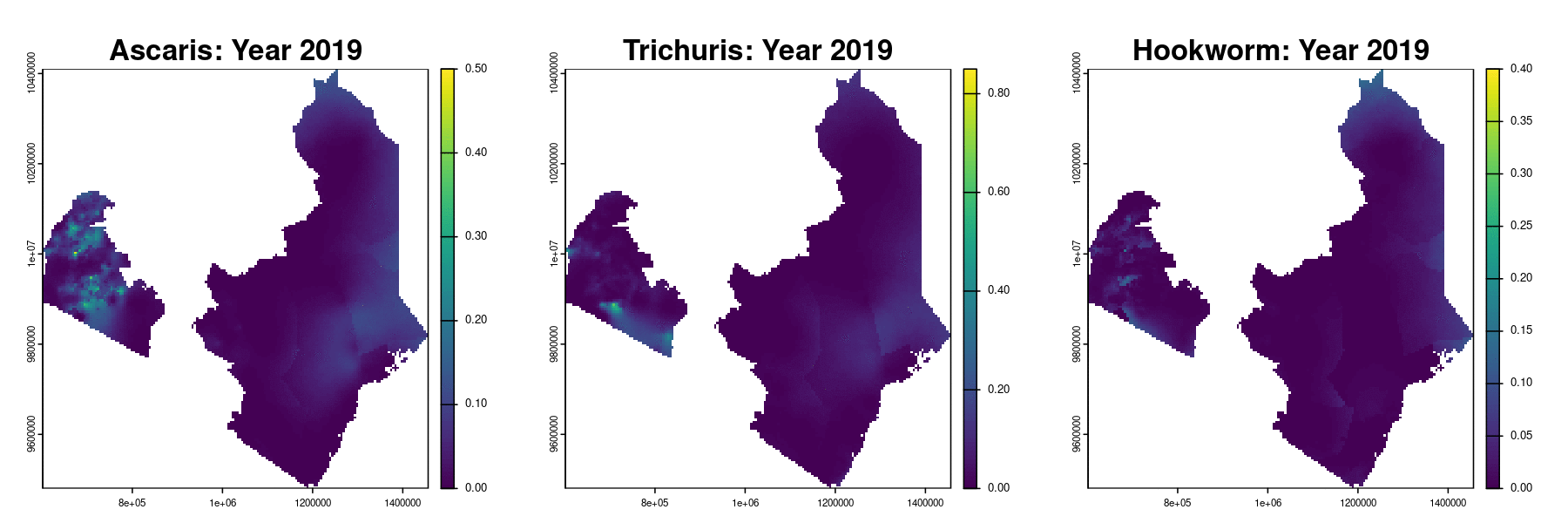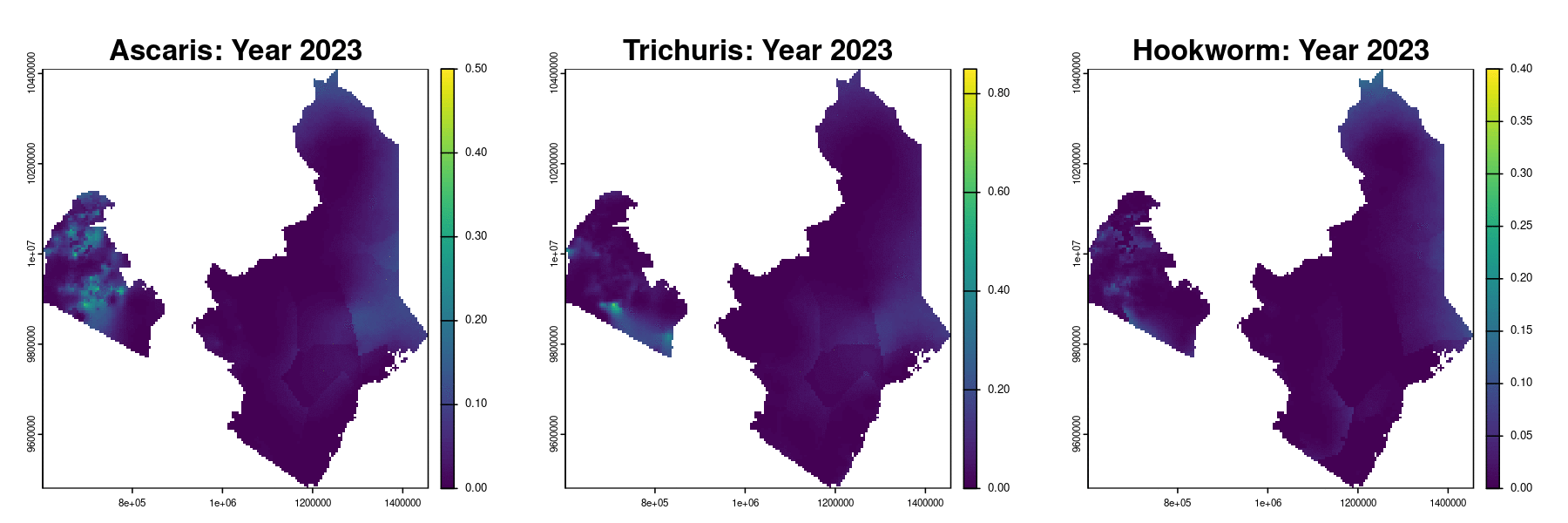
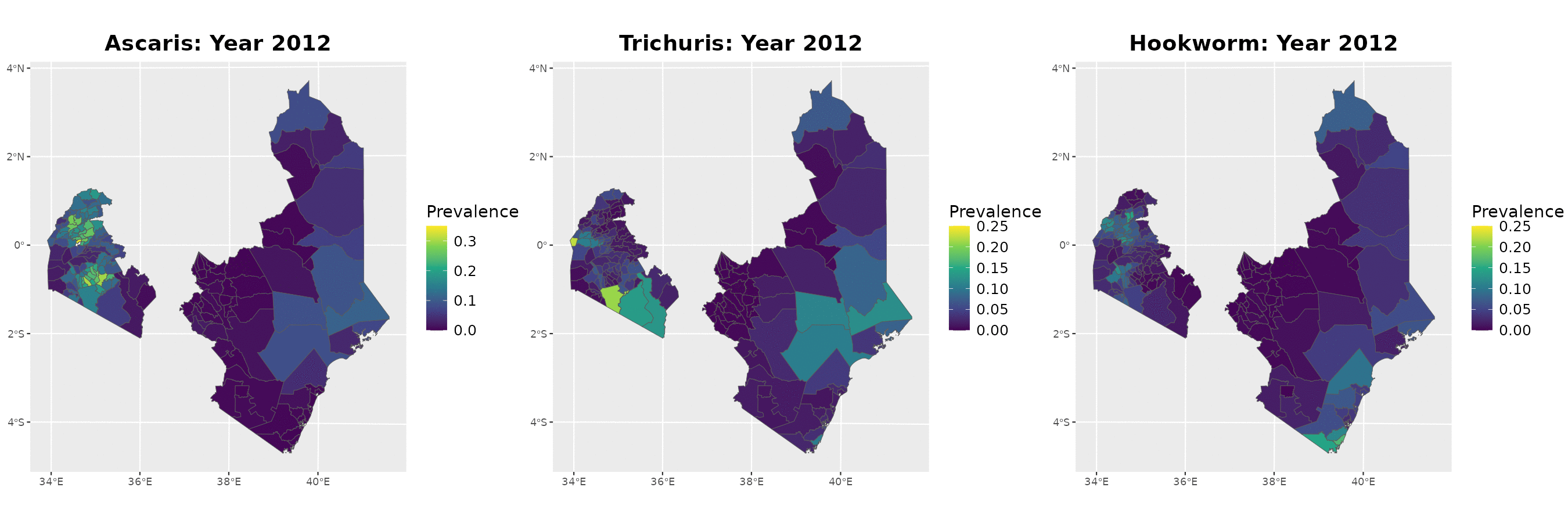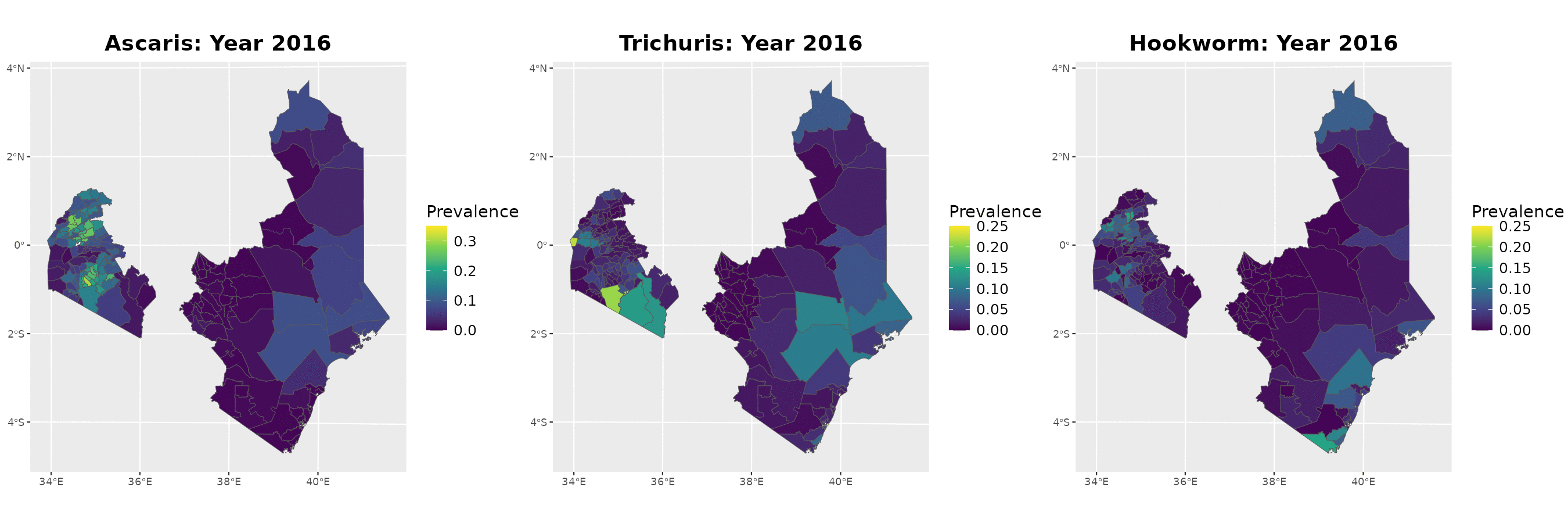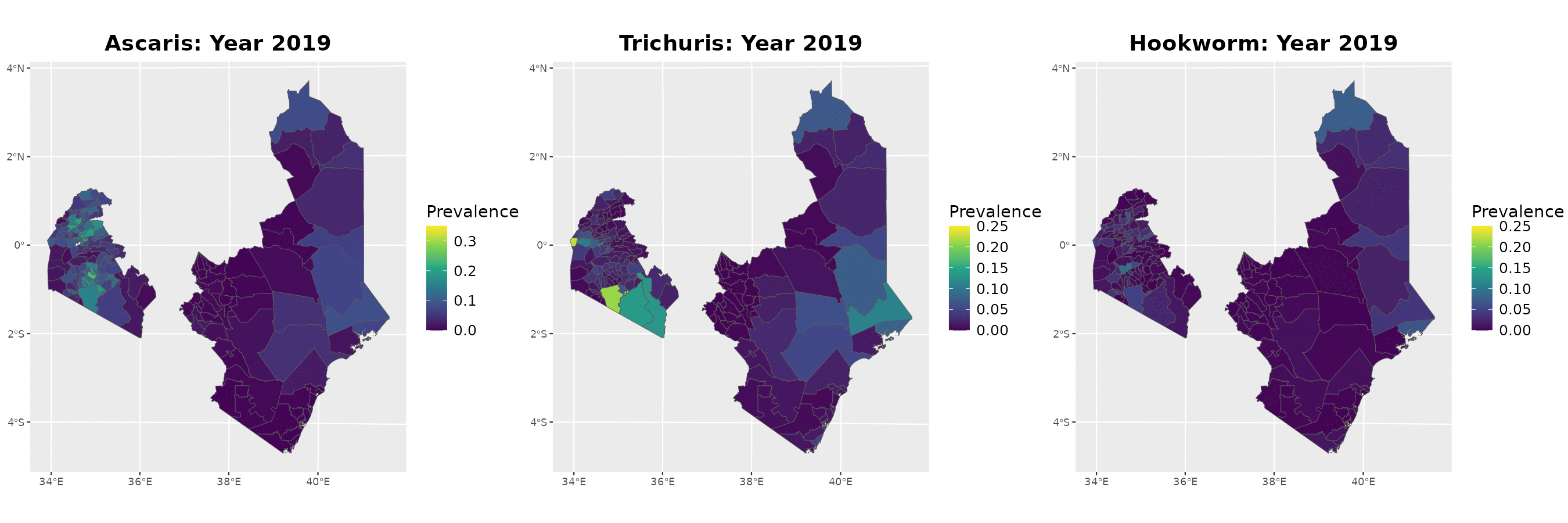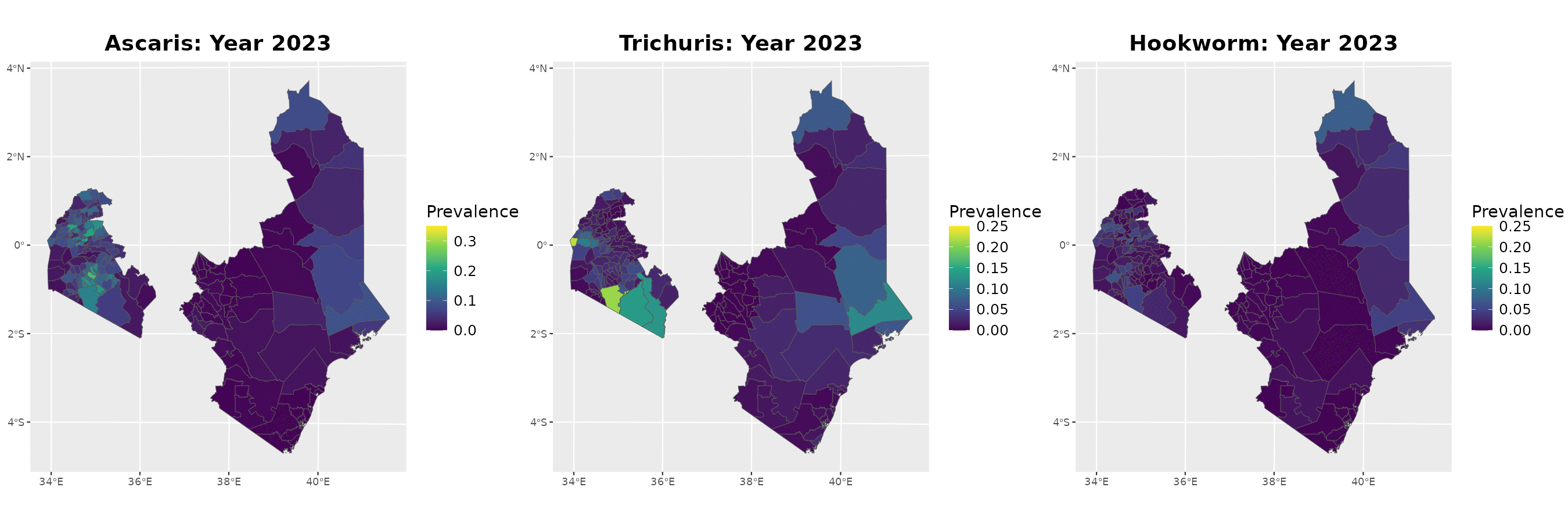
Next steps and extenions
Need for extension that includes \(S(x,t)\) rather than \(S(x)\).
Apply DAST to other NTDs.
Key assumptions of DAST
The MDA effect is the same across time
Incorporating data from multiple diagnostics
Accounting for the effect of different types of treatments
Uncertainty in the MDA coverage
THANK YOU!

🔗 giorgistat.github.io
📧 e.giorgi@lancaster.ac.uk
📍 CHICAS, Lancaster Medical School, Lancaster University, UK
